CBN Wheels
Eagle’s CBN Wheels
At Eagle Superabrasives we have harnessed the remarkable qualities of synthetic CBN crystals to engineer high performance grinding wheels that are perfect for grinding ferrous materials. Cubic Boron Nitride, rivaling diamond in hardness, forms the foundation of these cutting-edge wheels, enabling the aerospace, automotive, and tool manufacturing industries to achieve unparalleled levels of efficiency and durability. These CBN wheels exhibit exceptional thermal stability and hardness, which translates to the ability to machine hard materials with unprecedented precision, while minimizing heat generation.
EAGLE’S CBN BOND SELECTION
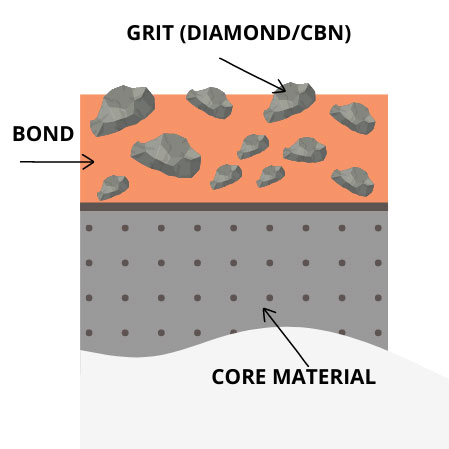
Our tried and true bond systems lock in the carefully selected CBN powders to ensure almost zero CBN pull out. The ingredient selection process provides a mechanical and chemical bond of the CBN particles to the bond system. This produces a long lasting, sharp and durable grinding wheel.
The Hardness of CBN Wheels
CBN grinding wheels are graded by the hardness of the bond. A higher-hardness bond allows for longer wheel life when compared to conventional wheels, such as aluminum oxide and silicon carbide.
To determine the hardness of a wheel, one must first understand how the abrasive grit works.
A wheel is considered soft if the abrasive grit fractures or pulls out from the bond easily. A wheel is considered hard when the abrasive grit is held into the bond firmly, and does not result in microfracture.
Typically, if the working material is easy to cut, a high-hardness wheel is suggested. A softer wheel is used for a more difficult to grind materials, such as sub micro-grain TCT cutting tools.
The Importance of the Bond
The bond of a CBN wheel is one of the most important factors when choosing the correct wheel for the job. Our unique bond system retains the CBN particle longer than most other bond systems, providing a longer wheel life and excellent wheel durability and special heat-reducing processes. We offer a variety of bonds for our CBN grinding wheels including Phenolic Resin, Polyimide Resin, metal bond, vitrified bond and hybrid bonds – all of which can be custom made to fit your needs.
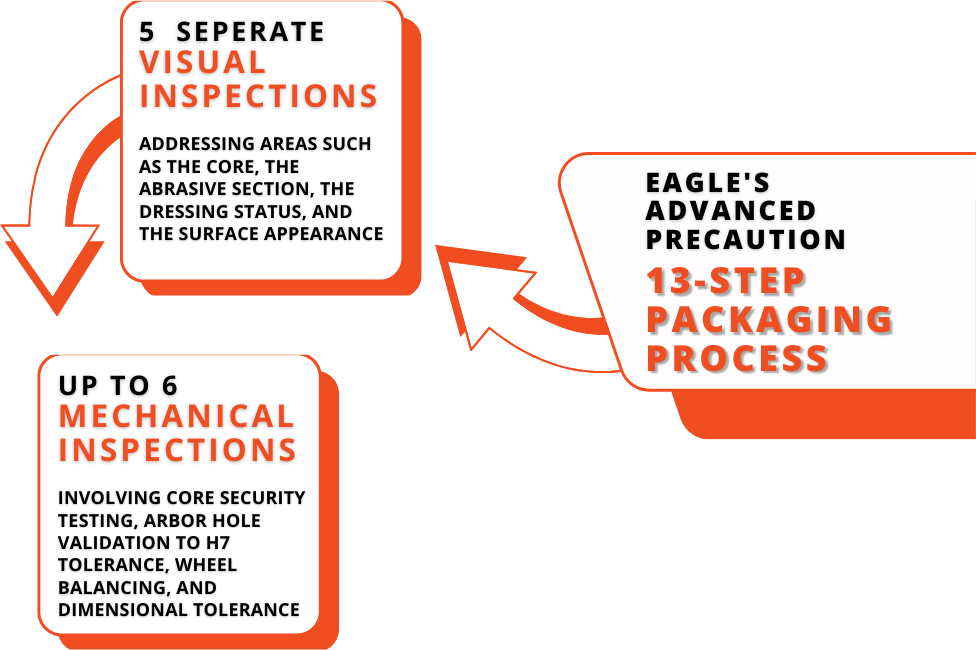
Eagle’s Shipping Process
At Eagle, we pride ourselves on providing exceptional solutions to your most common issues. Beyond delivering a product, we eliminate shipping issues by partnering with trusted logistics providers, guaranteeing reliable delivery, on time. Our CBN Abrasive WHEELS are engineered to your needs, optimizing performance and enhancing productivity. By investing in our solutions, many clients report extended durability and reduced maintenance costs.
Choose Eagle Superabrasives and gain a partner dedicated to providing advanced customer service and expert guidance. Our team of problem solvers is ready to help you optimize PRODUCTION. Experience the difference with us and unlock the full potential of your OPERATION.
About CBN Wheels
CBN is a man-made superabrasive that outperforms diamond when used for ferrous (iron-rich) grinding operations. CBN derives its name from its chemical makeup (cubic boron nitride). When working with ferrous (iron-rich) materials, CBN is the superabrasive of choice. CBN is a highly-durable, synthetic abrasive mineral. This type of grinding wheel works best with steel or cast iron application. When coupled with high temperatures, CBN is of an unreactive nature, making it the preferred choice for “heated situations”. Because of this, CBN wheels can be used at high speeds without affecting the life of the wheel.
CBN grinding wheels are used to sharpen, cut, or remove metal from hard surfaces, providing a consistent and even finish. Our CBN wheel can accurately cut metals, glass, and even diamonds. Most notably CBN wheels are used for hard, high-speed steels like woodworking tools, blades, bits, and cutters. When you’re tools become dull over time from use, a CBN grinder wheel will bring them back to life.
If you need a grinding wheel that will last longer, improve your operations, and comes with dedicated support, look no further than Eagle Superabrasives.

An Industry Leader
With Decades of Experience
Why Buy
From Eagle?
Trust the Experts
When you’re ready to try the best, trust the experts at Eagle Superabrasives. We offer unparalleled customer support and service.
With an extensive inventory of over 6,000 diamond, CBN and CDX wheels in stock at our North Carolina location, we are capable of shipping 90% of orders same day. Custom orders can be shipped in as little as seven days.
Our experienced staff is dedicated to providing our customers with high-quality products and excellent service. We offer technical support and advice to assist you along the way.
your grinding wheel needs.
When you’re ready, contact us for a free quote
or call us at (828) 759-5898